As of July 1, 2023, label claims on cattle steroid implant products were updated by their respective manufacturing companies to meet a request from the FDA to provide clarification on reimplanting use within a production phase. There are currently 25 approved implants for use in beef production in the U.S.
Three main types of hormones are used in cattle ear implants: androgens, estrogens and progestins. Estrogens consist either of the naturally occurring estradiol-17β, a synthetic version called estradiol benzoate or an estrogen-like compound called zeranol. Estrogens can be used either alone or in combination with the naturally occurring progestin, progesterone, or a synthetic version of testosterone, trenbolone acetate. Implants vary in potency and are commonly classified as low-, medium- or high-potency implants depending on their active ingredients and the concentration of their active ingredients (Table 1). Less potent implants usually contain an estrogenic hormone only or an estrogenic hormone with progesterone. Implants with medium potency usually contain an estrogenic hormone with a lesser concentration of an androgenic hormone, while higher potency implants contain an estrogenic hormone in combination with a greater concentration of trenbolone acetate.
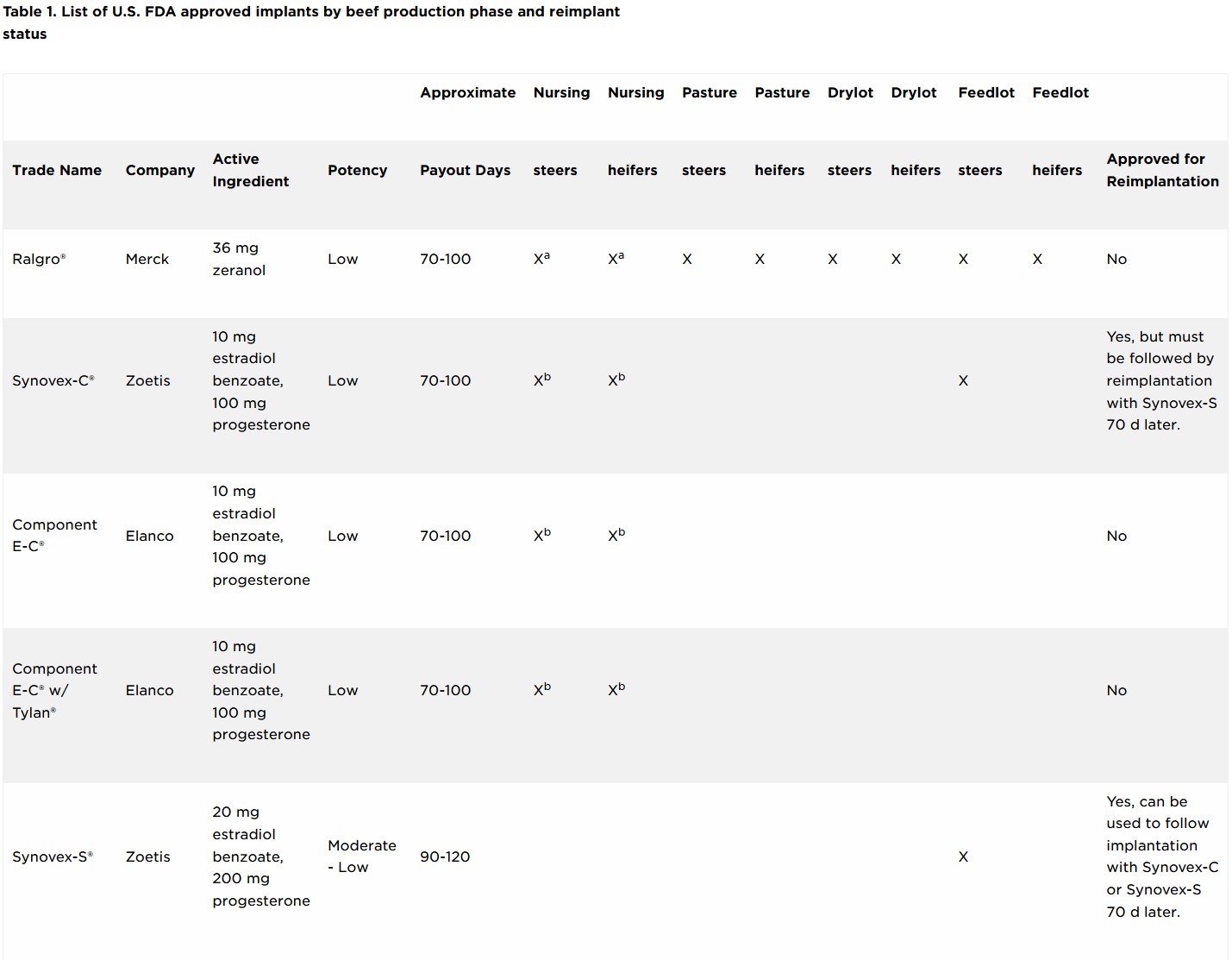
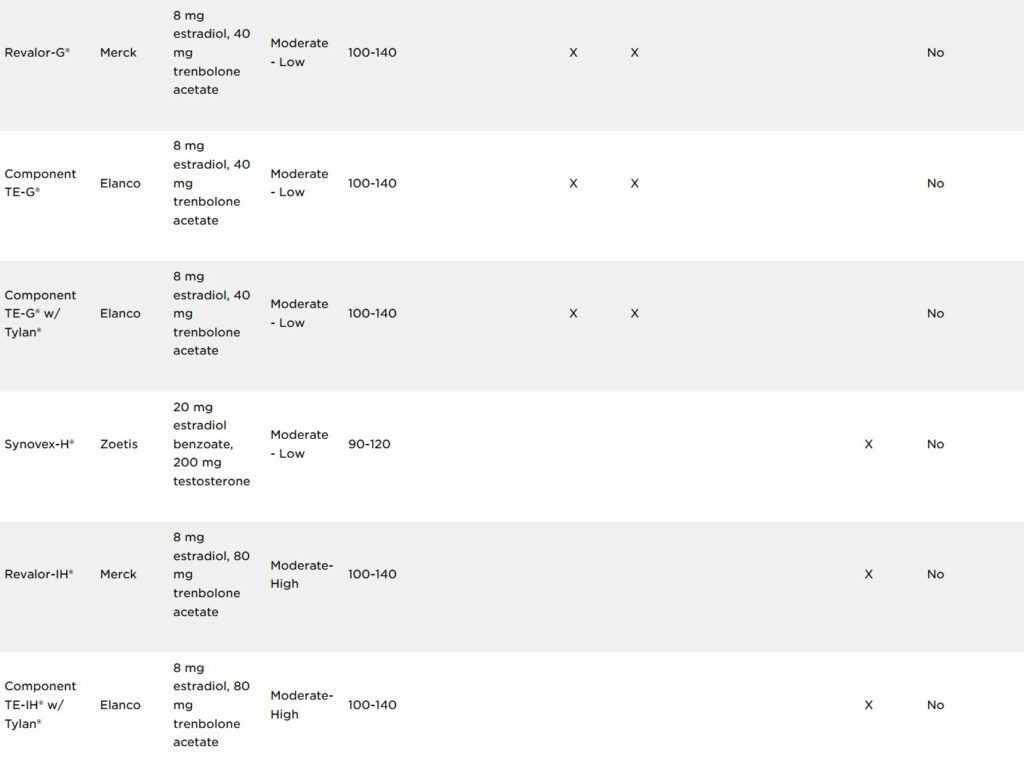
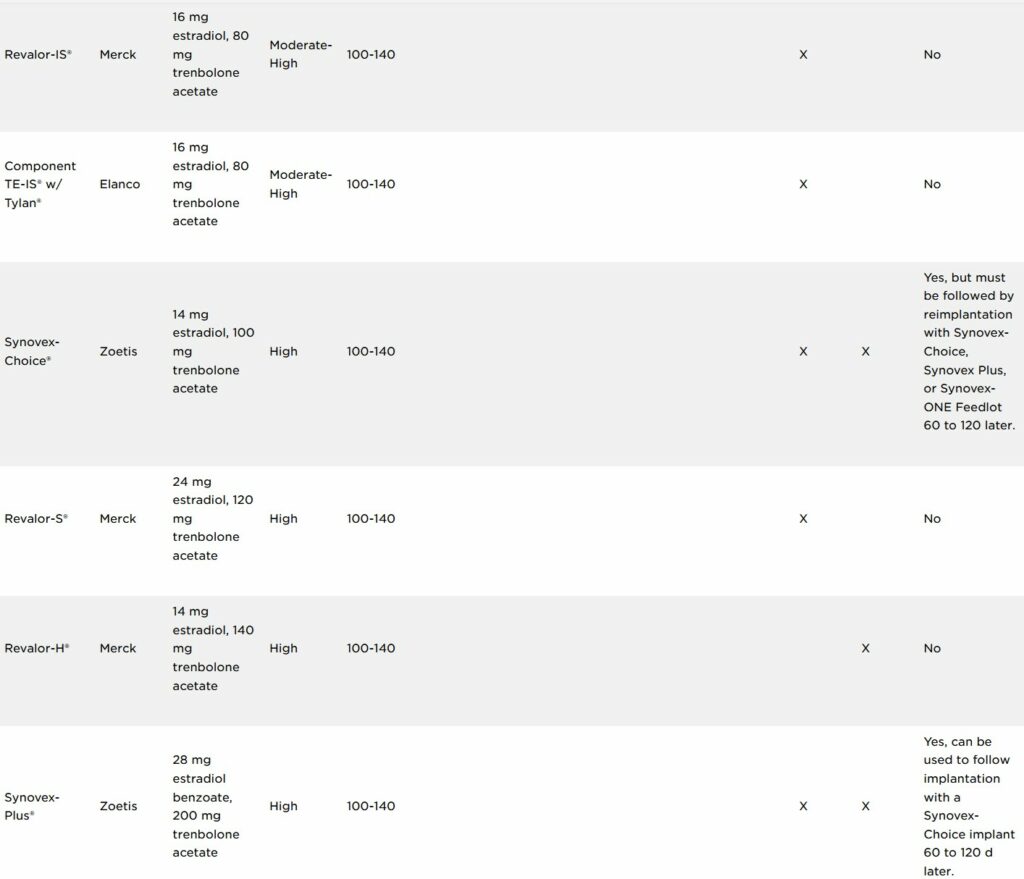

The expected time the active ingredients are released from the ear implant into the blood circulation of the animal is referred to as “payout.” Payout is the amount of time implanted cattle are expected to experience the benefits (i.e., increased average daily gain (ADG), improved feed efficiency and increased carcass leanness) of the steroid implant compared with non-implanted cattle. Implants can also be classified as non-coated or coated depending on their design and intended payout period. Coated implants extend the payout period of the implant by slowing the release of the active ingredients from the pellets within the implant. Of the 25 approved implants currently in use, six are coated to achieve an extended payout period. Coated implants require less cattle handling and processing for reimplanting cattle to continue achieving the beneficial effects of the implant.
Steroidal ear implants are a growth promoting technology administered subcutaneously in the back of the middle one-third of the ear to increase growth, feed efficiency and carcass leanness of beef cattle. As a result, implanted cattle typically achieve a greater final body weight before slaughter due to the increased muscle deposition and delayed fat deposition. The negative effects observed with the use of implants are possible reductions in tenderness and/or marbling deposition. Tenderness differences can be mitigated by postmortem aging (i.e., dry or wet aging), at least 14 or 21 days. Implanted cattle typically require an additional 90 to 110 pounds live body weight to achieve similar marbling scores compared with non-implanted cattle. Many factors such as breed, diet, implant selection and timing can influence the expected response from implants. It is recommended that you speak with your regional implant sales representative or beef extension educator about developing an appropriate implant strategy for your operation.
Cattle producers raising cattle for terminal beef production can choose to administer implants at one or more of the various beef production phases recognized by the U.S. Food and Drug Administration’s Center for Veterinary Medicine (FDA-CVM). Implants have been approved by the FDA for safe and effective use in the beef production phases listed below.
Approved beef production phases for implant use by U.S. FDA (Guidance for Industry #191)
- Beef calves 2 months of age and older – beef calves considered ruminating and nursing their dams from 2 months of age to weaning.
- Growing beef steers and heifers on pasture (stocker, feeder and slaughter) – weaned growing beef steers and heifers (beef and dairy breeds) intended only for slaughter (i.e., not for reproductive purposes) maintained on pasture and receiving a majority of their diet from grazing.
- Growing beef steers and heifers in a dry lot – weaned growing beef steers and heifers (beef and dairy breeds) that receive harvested forages as a majority of their diet and are reared on dormant pastures with insufficient biomass to sustain typical growth and/or housed in dirt floor pens. Cattle in this production phase may receive minimal supplementation, such as a protein supplement, to achieve growth rates consistent with those typically observed in cattle on pasture.
- Growing beef steers and heifers fed in confinement for slaughter – weaned growing and finishing beef steers and heifers (beef and dairy breeds) intended only for slaughter (i.e., not for reproductive purposes) and confined in group pens and fed a progressively high-energy diet ad libitum as their sole ration until slaughter. May also be referred to as feed yard or feedlot cattle in the industry. Includes growing beef steers and heifers in a grow yard (see definition below).
- Growing beef steers and heifers in a grow yard – a subset population of growing beef steers and heifers fed in confinement for slaughter, these are weaned growing beef steers and heifers (beef and dairy breeds) confined in group pens and fed a moderate- to high-roughage diet ad libitum as their sole ration prior to the finishing stage. Grow yards may also be referred to as starter yards or backgrounding yards in the industry and may be co-located on a feedlot or housed at a separate facility.
Implant labels will clearly state which production phase(s) it is approved for, and if it is approved for reimplantation within a particular production phase. The right side of Table 1 identifies which implants are approved for reimplantation within a production phase.
While implant use is approved for Beef calves 2 months of age and older and Growing beef steers and heifers on pasture (stocker, feeder, and slaughter), cattle cannot be reimplanted (i.e., receive two implants) in either of these production phases. At this time, a select number of implants approved for use in Growing beef steers and heifers fed in confinement for slaughter are the only implants approved for reimplantation.
Growing beef steers and heifers in a Grow Yard production phase is a subset classification of Growing beef steers and heifers fed in confinement for slaughter. Think of this as a smaller set of a larger population, basically the receiving/growing cattle in the feedlot. The Growing beef steers and heifers in a Grow Yard production phase was designed with the intent for drugs to be labeled for these cattle, separate from cattle in the Growing beef steers and heifers fed in confinement for slaughter production phase. No implants are specifically labeled for approved use in the Growing beef steers and heifers in a Grow Yard production phase. Implants would be labeled for approved use in cattle in the Growing beef steers and heifers fed in confinement for slaughter production phase instead, which also includes cattle in the Growing beef steers and heifers in a Grow Yard production phase.
Implants with Tylan (i.e., some Component brand implants) will require authorization from your veterinarian for purchase and use because of the antibiotic properties Tylan offers to provide protection against potential ear abscess development after implant administration. Also, be aware that veterinarians cannot prescribe extra-label use of implants for growth-promoting purposes because they are not being used for the treatment of disease.
Lastly, confusion remains regarding the difference between a dry lot and grow yard, even after the FDA’s attempt to clarify the definition of a dry lot. Both phases confine cattle to pens before entering the feedlot. A dry lot specifies dirt floor pens (or dormant pasture) while a grow yard does not specify the flooring. In many feedlots across the U.S., feedlots may consist only of dirt floor pens, while other feedlots may have solid or slatted concrete floors. Both phases require cattle to be fed a diet consisting of primarily harvest forages, common of a backgrounding/receiving/growing diet prior to feedlot entry. In both phases a supplement is allowed to provide a balanced diet, considering protein, mineral and vitamin requirements. Location does not matter either, as cattle in the grow yard phase can be located on or off site of the feedlot. The FDA could provide more clarification on the difference between these two production phases in the future.
Table 2. Example of feedlot performance and economic return for implanting one steer
| Non-Implanted | Implanted | Difference (Implanted minus non-implanted) | |
| Performance | |||
| Days on feed (d) | 200 | 200 | – |
| Average daily gain (lb/d) | 3.50 | 4.03 | 0.52 |
| Total weight gain (lb) | 700 | 805 | 105 |
| Dry matter intake (lb/d) | 22.0 | 23.1 | 1.1 |
| Total dry matter intake (lb) | 4400 | 4620 | 220 |
| Feed:Gain (lb DM:lb gain) | 6.29 | 5.74 | -0.55 |
| % USDA Choice or greater | 85% | 70% | -15% |
| Prices | |||
| Implant(s) per animal | $0.00 | -$8.00 | -$8.00 |
| Processing fee per animal | $0.00 | -$2.00 | -$2.00 |
| Feed cost ($/lb DMB) | -$0.18 | -$0.18 | – |
| Choice live price ($/lb) | $1.80 | $1.80 | – |
| Select live price ($/lb) | $1.65 | $1.65 | – |
| Choice dressed price ($/lb) | $2.90 | $2.90 | – |
| Select dressed price ($/lb) | $2.66 | $2.66 | – |
| Select carcass discount ($/lb) | -$0.24 | -$0.24 | – |
| Returns | |||
| Choice weight gain ($)1 | $1,071.00 | $1,014.30 | -$56.70 |
| Select weight gain ($)2 | $173.27 | $398.51 | $225.24 |
| Dry matter intake ($) | -$792.00 | -$831.60 | -$39.60 |
| Implanting cost ($) | $0.00 | -$10.00 | -$10.00 |
| Total return ($) | $452.27 | $571.21 | $118.94 |
| 1 Total weight gain × Choice live price × % grading USDA Choice or greater | |||
| 2 Total weight gain × Choice live price × % grading less than USDA Choice | |||
For cattle feeders questioning whether to use implants or not, consider the economic incentive. In this example, consider a 15% increase in ADG and 5% increase in dry matter intake (DMI) in implanted compared with non-implanted cattle. Implanted cattle typically have a greater DMI to support their additional growth. Let us say that the non-implanted cattle have an ADG of 3.50 lbs./day and DMI of 22.0 lbs./day, and implanted cattle have an ADG of 4.03 lbs./day and DMI of 23.1 lbs./day over the course of a 200-day feeding period (Table 2). During this length of feeding period, one to two implants could be administered for approximately $8 per animal. If implant administration is not conducted when other processing is done (e.g., vaccines, antimicrobial treatment), a cost of $2 per animal for processing the animal through the chute an extra time is warranted. Consider a live market price of $1.80/lb. for cattle grading USDA Choice, feed cost of $0.18/lb. feed dry matter and a USDA Select quality grade discount of $0.2417/lb. for a live market price of $1.65/lb.
Marbling deposition can be less for implanted cattle fed for a similar number of days; therefore, implanted cattle in this example had 15% fewer cattle grading USDA Choice or greater. Implanted cattle will have gained an additional 105 lbs. live weight and consumed an additional 220 lbs. of dry matter compared with non-implanted cattle. Implanted cattle will have earned $128 per animal more than non-implanted cattle. Subtract the cost of implants and the return on your investment (ROI) of using implants is $118 per animal or approximately 12:1. If you are not implanting your cattle intended for slaughter, does your non-implanted market allow you to capture this additional value gained from using implants?
SOURCE: Jerad Jaborek, Michigan State University Extension – August 10, 2023
PHOTO: Jerad Jaborek











